
Why does microfibrillated cellulose tolerate salts so well?
Anni Karppinen | June 27, 2017
Microfibrillated cellulose (MFC) differs from many rheology modifiers in that aspect that it can be used in high salinity formulations. The rheology effect comes from entangled fibers and salts do not influence this network as it does when the rheology effect is based on ionic interactions. However, the viscosity and other rheological properties vary slightly as a function of salt concentration. Let’s take a closer look at the reasons behind this.
Salt affects the interactions between the fibers
MFC consists of small fibers which form a three dimensional network in water suspension. This network is based on physical interactions between the fibers which makes it very strong and robust towards changes in the chemical environment, like salt concentration (see also how pH affects the network here). This behavior differentiates MFC from many polymeric rheology modifiers which may be sensitive to salinity since salt concentration changes the conformation of the polymer chain or even the solubility. For example, the water solubility of alkali-soluble emulsions (ASE) and hydrophobically-modified alkali-soluble emulsions (HASE) are based on the anionic groups on the polymer. Salt screens these charges, thus reducing the solubility of the polymers. This leads to a drastic drop in viscosity performance of such polymeric rheology modifiers.
Even if the rheological effect of MFC is not based on ionic groups, MFC fibers (as all cellulosic materials) also have slight negative surface charge which causes repulsion between the fibers. Adding a small amount of salt reduces the repulsion between the fibers and makes the network stronger. We can follow the fiber-fiber interactions by measuring the complex viscosity in an oscillation measurement (see Figure 1). Oscillation measurements are performed in steady state and they describe the material behavior at rest. The complex viscosity reaches a maximum at certain concentration (here at 0.35%) which means that the fibers are interacting the strongest at this salt concentration.
Increasing the concentration over this point results in a decreasing complex viscosity. This indicates that the interactions are so strong that the fibers start to form flocs instead of continuous network. The flocs have very strong interactions between the fibers but the interactions are somewhat lower between the flocs. Note that to be able to see the changes in the fiber-fiber interactions, the fibers need to be in water which allows the fibers to move relatively easily towards each other or away from each other. If MFC is included into a formulation, the other ingredients hinder the fiber movement and this kind of change in the complex viscosity is not seen.
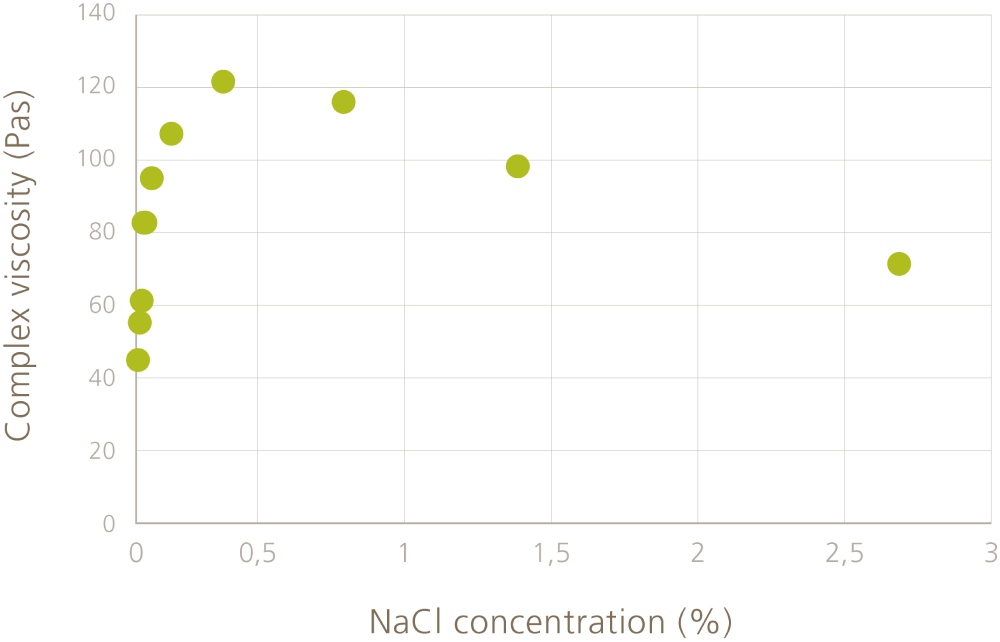
Figure 1. Complex viscosity of 1.4% MFC suspension (Exilva, Borregaard) as a function of salt (NaCl) concentration.
Viscosifying effect remains even at high salt concentrations
Complex viscosity is very sensitive to small changes in the fiber-fiber interactions. However, it does not mean that the flow viscosity (“normal” viscosity) changes as much. Figure 2 shows flow viscosities for MFC suspensions with high salinity. If we look at the flow viscosities of these MFC/salt suspensions, we see that it does not change so much with different salts.
This is because the overall fiber network is not just based on the charges. Instead, it is a physical network with entangled fibers and these entanglements do not disappear even if the interactions between the fibers change slightly with salt. Under flow, the network breaks into smaller fragments (flocs) and we observe the effect of these flocs as flow viscosity. Floc size changes a bit based on the type of the salt and salt concentration and that is why we see some differences between the different salts but generally the viscosifying effect remains.
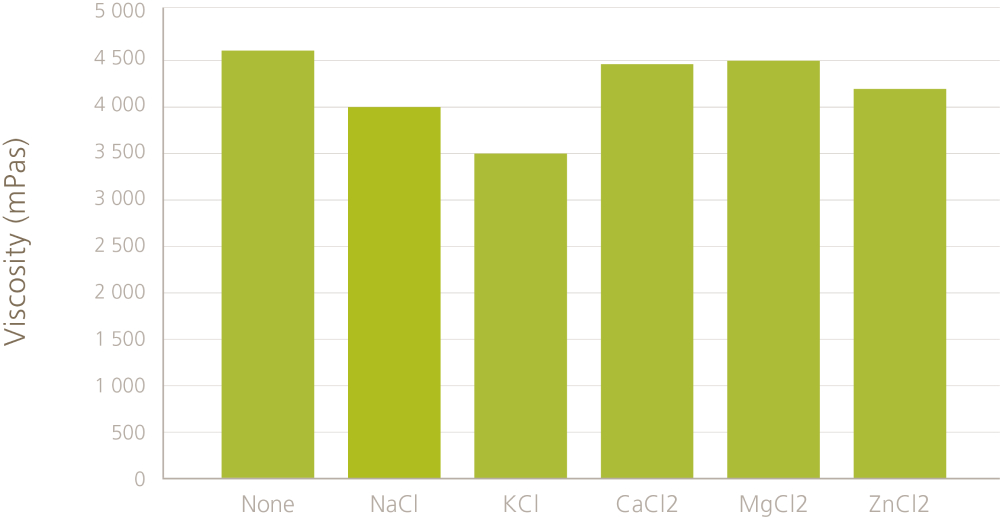
Figure 2. Flow viscosities of 1% MFC in salt solutions. The concentration of salts is 10% (w/w). Viscosity is measured by Brookfield viscometer with spindle V-72 after 5 min at 10 rpm.
MFC network tolerates salts very well and it can be used as a rheology modifier in high salinity formulations, where the options otherwise may be limited. However, some changes in the floc structure will appear, especially when looking at MFC/water suspensions. In practice, this is often irrelevant since MFC is mixed with other substances. However, it is important to remember that this can affect your viscosity or other measurements if you are performing them in water.
Written by:
Anni Karppinen
