
I am looking to improve my rheology in difficult ph ranges: where do I look?
Anni Karppinen | June 19, 2018
Controlling rheology in very acidic or alkaline environments can be a tricky challenge. Different products ranging from examples like concrete to, your household cleaning products, are very different on pH. Extreme pH conditions can decrease the efficiency and functionality of some rheology additives, but there are some available alternatives. This post will try to give you some ideas on how the technology of cellulose fibrils can interact in your formulation and make sure you stay in control over your product´s rheology under difficult circumstances. And why is this ability from the cellulose giving you this benefit?
Strong network of fibers
The rheological properties of microfibrillated cellulose ( in short MFC, part of cellulose fibrils products) are based on the strong three dimensional network of cellulosic microfibers. Cellulose does not dissolve or degrade even in extreme pH conditions, which is related to its high crystallinity and strong molecular chain-to-chain affinity. This makes it different from rheology modifiers like HASE (hydrophobically modified alkali-swellable emulsions) and HEUR (hydrophobically modified ethylene oxide urethane).
In addition, the cellulose fibrils like this are mainly connected via physical entanglements which are not sensitive to changes in pH. These two factors make cellulose fibrils stable in alkaline, neutral and acidic conditions.
The graph below illustrates the complex viscosity for 1% cellulose fibrils suspension in water at high, close to neutral and low pH over time. The viscosity does not change during 12 weeks, showing that the fiber network is intact. However, the absolute value of viscosity is dependent on the pH. let us look at the reason for that.
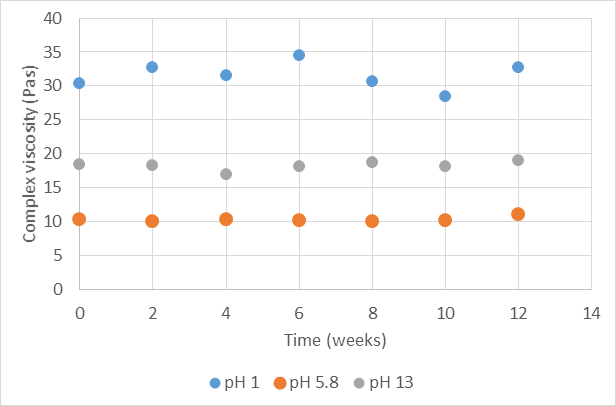
Complex viscosity for 1% cellulose fibrils (Exilva, Borregaard) in water at different pH. The suspensions are stable over 12 weeks at pH 1, 5.8 and 13.
Surface charge and pH
Cellulose fibrils is stable at different pH conditions, but the network strength is dependent on pH. Cellulose fibrils has slightly negatively charged surface which causes repulsion between the fibers. Even if the network of microfibrils is mainly based on the physical entanglements, these surface charges contribute to the overall strength of the network.
The charges are mainly due to occasional carboxyl groups on the fiber surface. Carboxylic groups are weak acids. The higher the pH, the more of them are dissociated and have negative charge.
In addition, the hydroxyl groups on the cellulose dissociate at very high pH. This means that the surface charge of cellulose is dependent on the pH, which in turn means that the repulsion between the fibers is also dependent on the pH. (More information about charges on cellulosic surfaces can be found, for example, in MT Goulet’s dissertation, 1989).
By measuring the viscosity of the cellulose fibrils suspension, we get information how strong the fiber network is. The graph below shows the viscosity of a cellulose fibril suspension at different pH. At very low pH, the viscosity is high since few carboxylic groups are dissociated, and the surface charge is low. This means that the repulsion between the fibers is small, and the network is very strong.
As the pH is increased, more carboxylic groups are dissociated, and the surface charge increases. This, in turn, lowers the viscosity since the connections between the fibers become weaker.
At very high pH the viscosity increases again which is presumably due to the improved dispersion of the fibers. However, even if the forces between the fibers change slightly with pH, the fiber network does not collapse, and it preserves its rheological and stabilizing properties at every pH.
In addition, the example here is given in pure water, and the changes are expected to be lesser in formulation where other components are present. If you are interested in reading more about the pH dependence of cellulose fibril viscosity, have a look at scientific articles by Pääkkö et al. (2007) or Ono et al. (2004), for example.
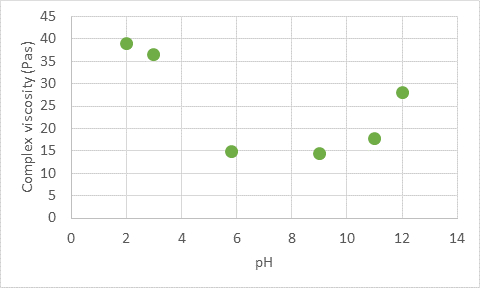 Complex viscosity of 1% cellulose fibril suspension (Exilva, Borregaard) as a function of pH.
Complex viscosity of 1% cellulose fibril suspension (Exilva, Borregaard) as a function of pH.
All in all, cellulose fibrils can be used as a rheology modifier and stabilizer in a wide range of pH since it does not degrade or dissolve even in very high or low pH. This bring about good opportunities for designing the manufacturing process or using the same rheology modifier for all your products.
→ Read also: Why Cellulose Fibrils is a completely new cellulose product
* This blog post was originally posted on June 28th, 2016, but we have updated it to give you the latest news and ideas on the topic.
Written by:
Anni Karppinen
